Back To Listings


- First authors: Young-Han Shin
- Corresponding authors: Suklyun Hong
- Whole authors: Young-Han Shin, Suklyun Hong
- Authors from M3L: Young-Han Shin
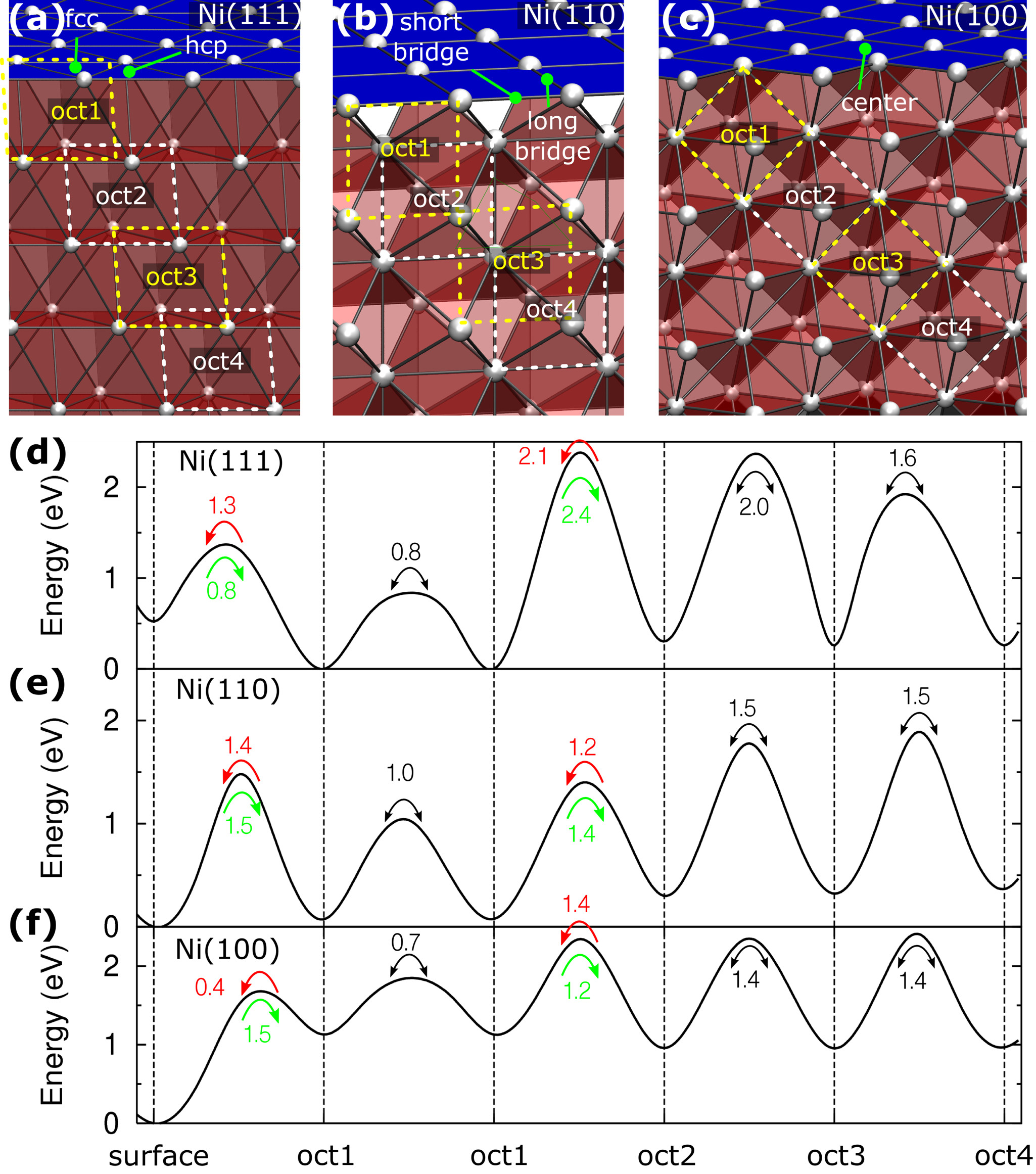
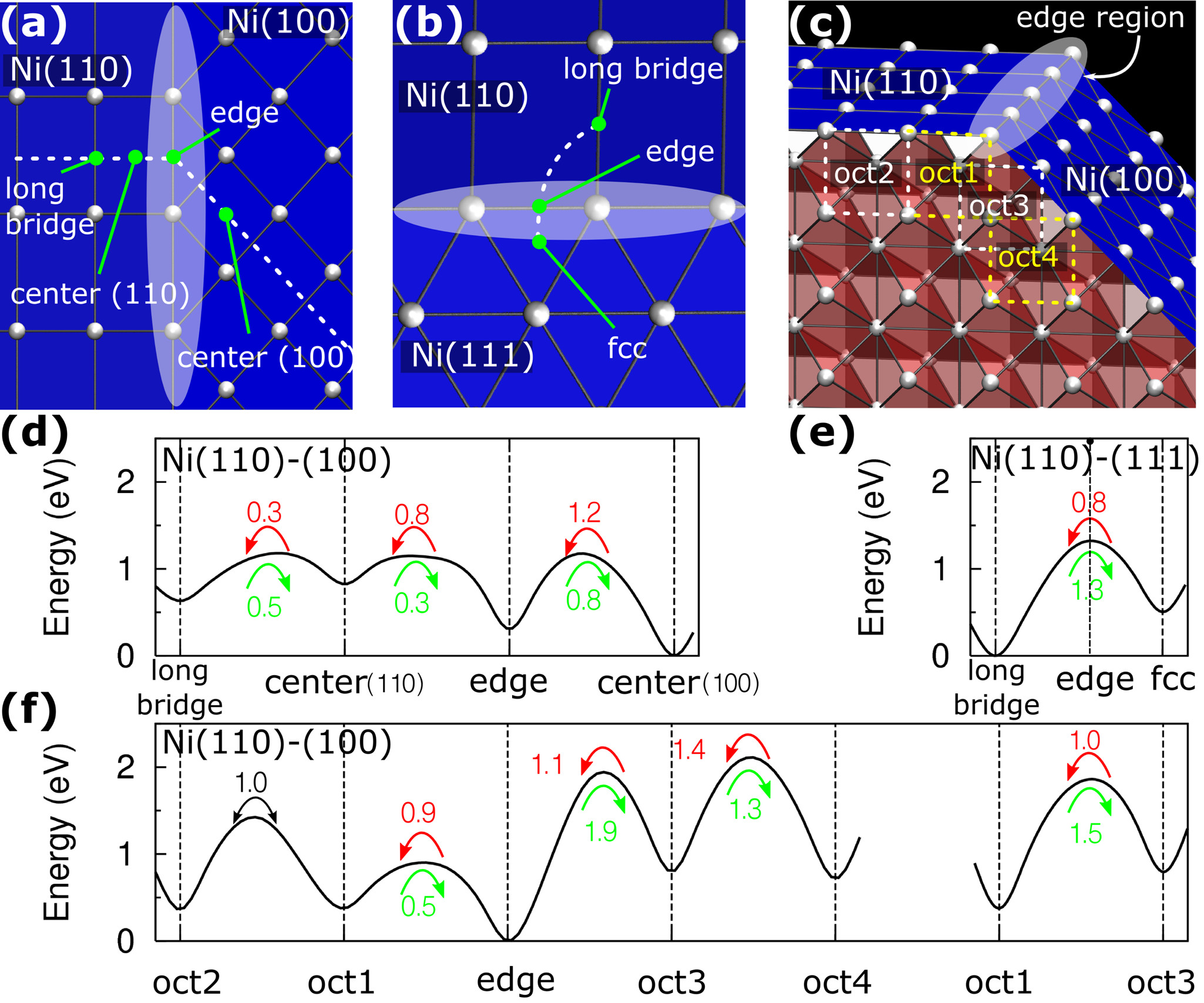
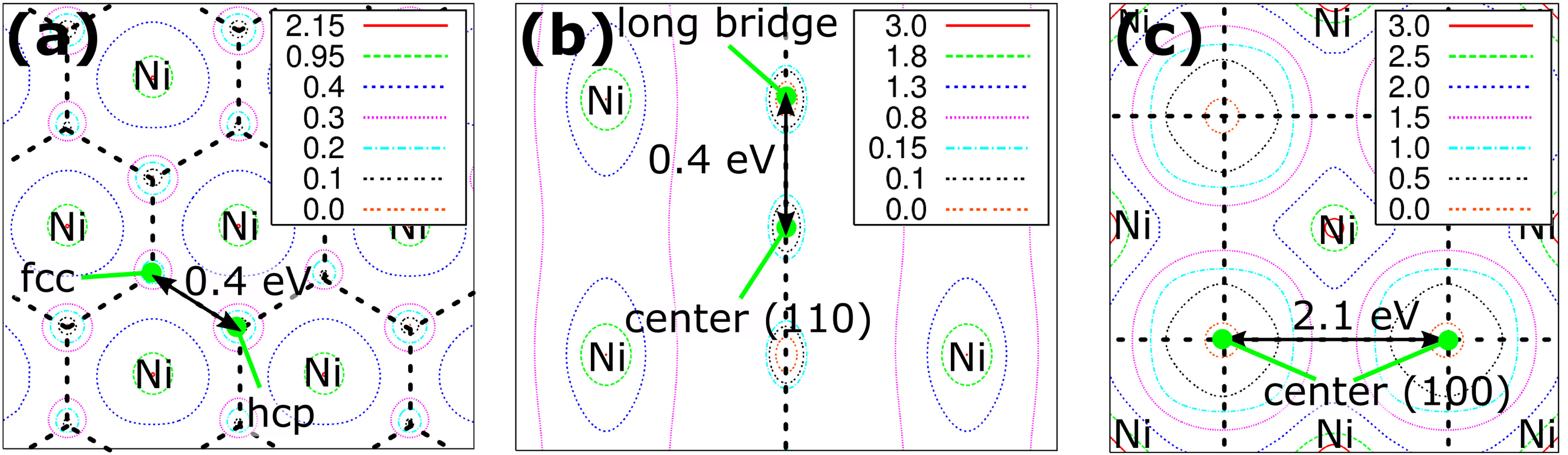
Adsorption and diffusion of carbon atoms in nickel are studied by density-functional theory calculations to understand the growth mechanism of carbon nanotubes in its initial stage. Combined with the results for surface and subsurface diffusions, the diffusion behaviors around the edges between low-index nickel surfaces reveal that the growth of carbon nanotubes is related to the diffusion barriers across these edge regions of nickel nanoparticles in addition to the growth temperature. We explain these results in terms of the reaction mechanisms which are kinetically or thermodynamically controlled depending on the temperature at which the carbon nanotubes grow.
Authors from M3L