Back To Listings




- First authors: Abdus Samad
- Corresponding authors: Young-Han Shin
- Whole authors: Abdus Samad, Mohammad Noor-A-Alam, Young-Han Shin
- Authors from M3L: Abdus Samad, Mohammad Noor-A-Alam, Young-Han Shin
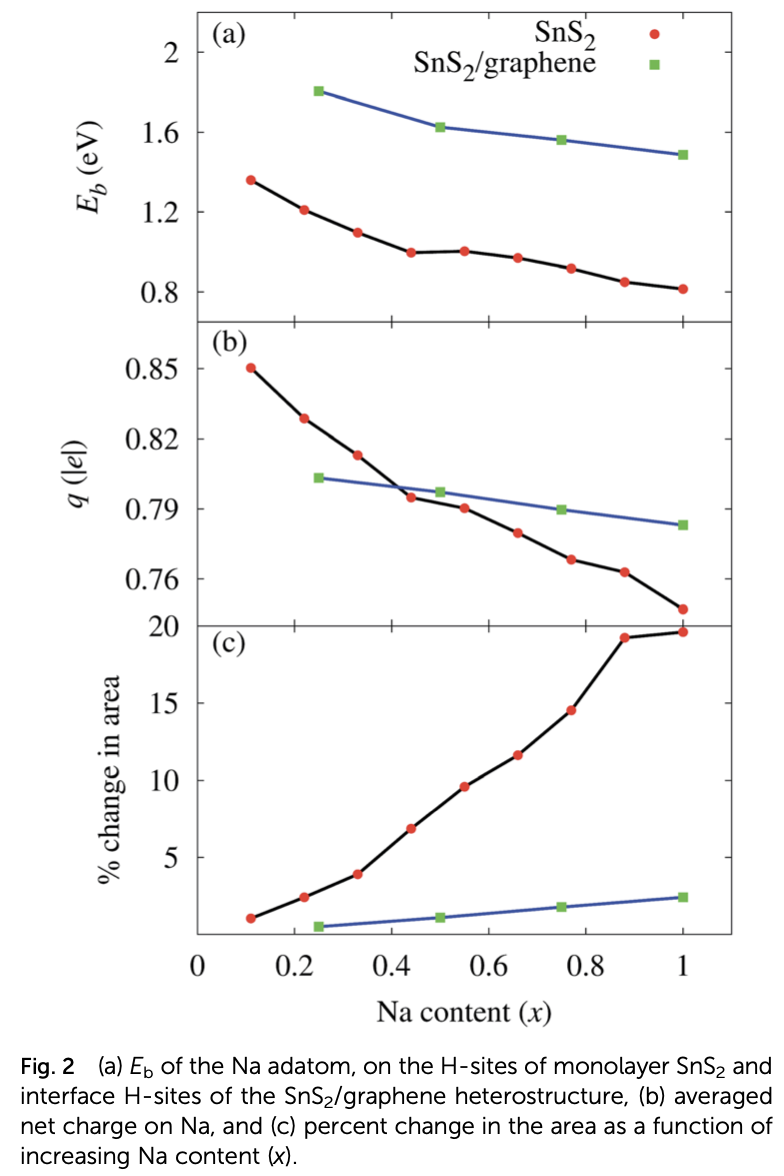
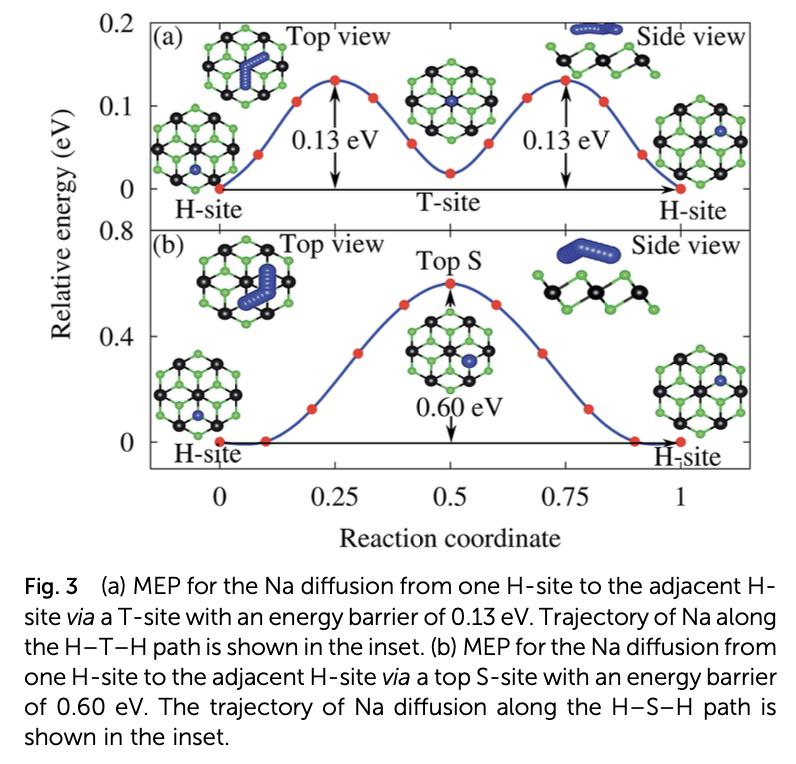
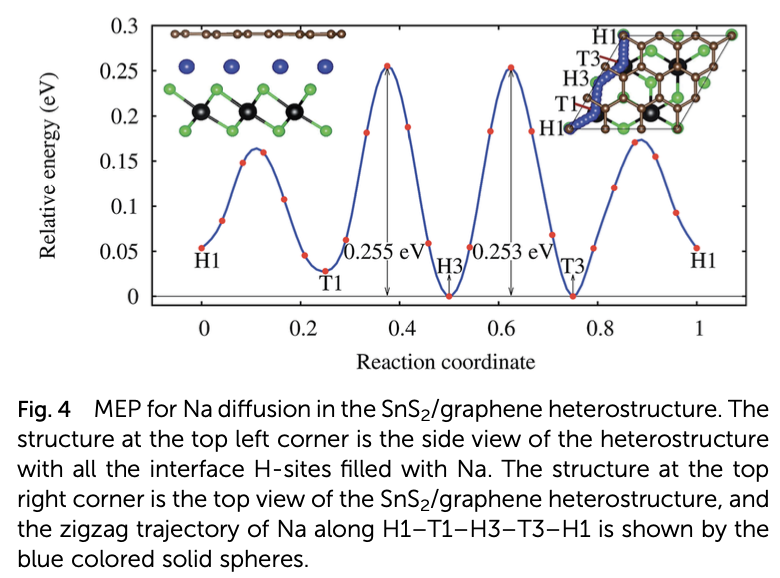
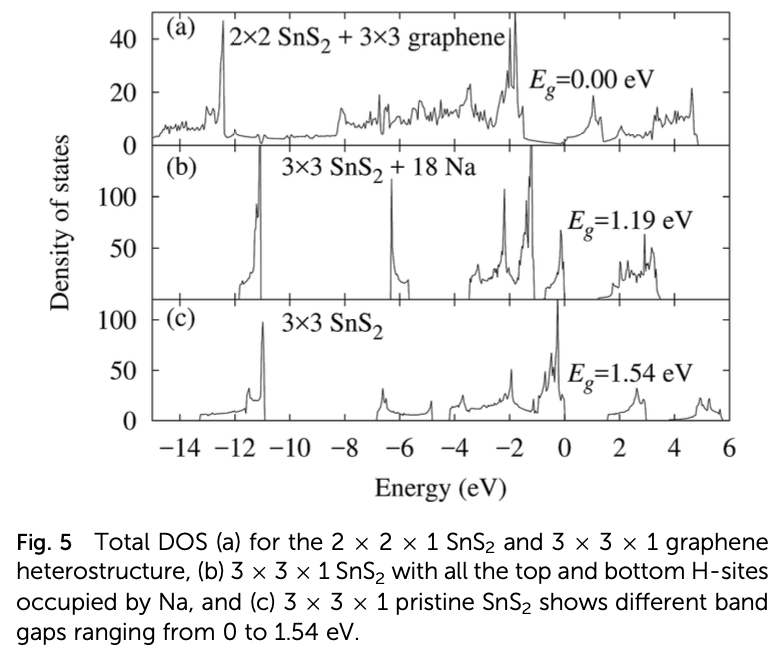
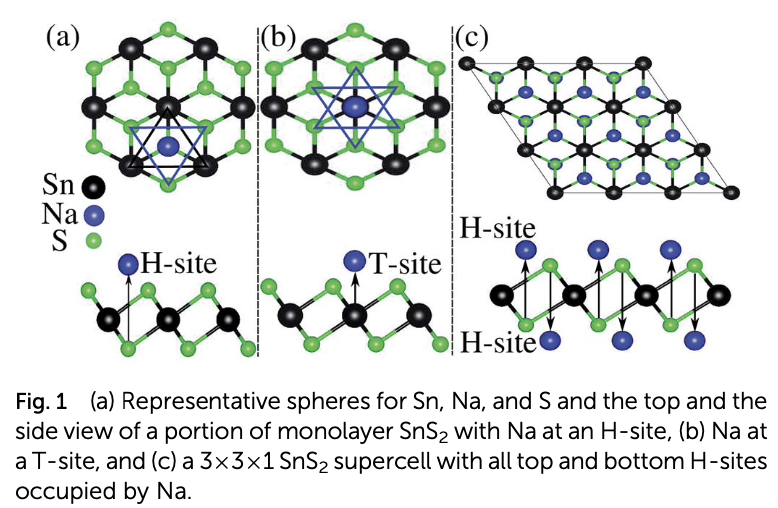
Properties such as the high binding energy of the Na adatom, high charge storage capacity, low half-cell voltage, and low activation energy barrier for Na diffusion render monolayer SnS2 a suitable anode material for rechargeable sodium ion batteries. However, the large expansion of the pristine monolayer SnS2 during sodiation and its high band gap, which is a barrier to the free flow of electrons, limit its practical use in batteries. These limitations can be adequately overcome by making a SnS2/graphene heterostructure. The graphene layer of the heterostructure prevents the SnS2 layer from expanding during sodiation and enhances its electrical conductivity, while the SnS2 monolayer makes Na atoms bind tightly. Even though the energy barrier for Na diffusion is increased by the heterostructure, it still competes with popular anode materials for Li and Na ion batteries. The combination of abundant and low-cost carbon, SnS2, and Na has high potential as an efficient commercial anode material for non- toxic rechargeable Na ion batteries.
Authors from M3L

Abdus Samad
samadstar143@gmail.com
Mohammad Noor-A-Alam
noor.uou@gmail.com