Back To Listings




- First authors: Abdus Samad
- Corresponding authors: Young-Han Shin
- Whole authors: Abdus Samad, Aamir Shafique, Young-Han Shin
- Authors from M3L: Aamir Shafique, Abdus Samad, Young-Han Shin
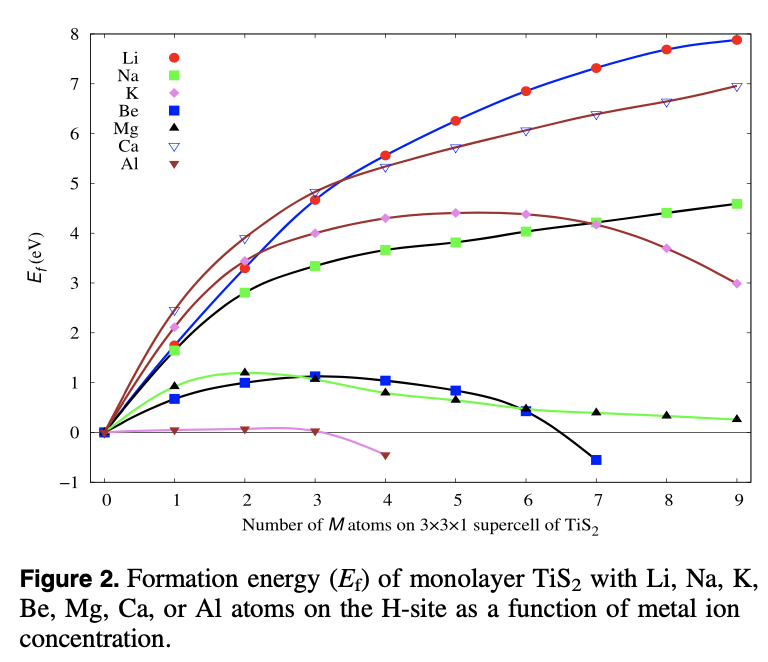
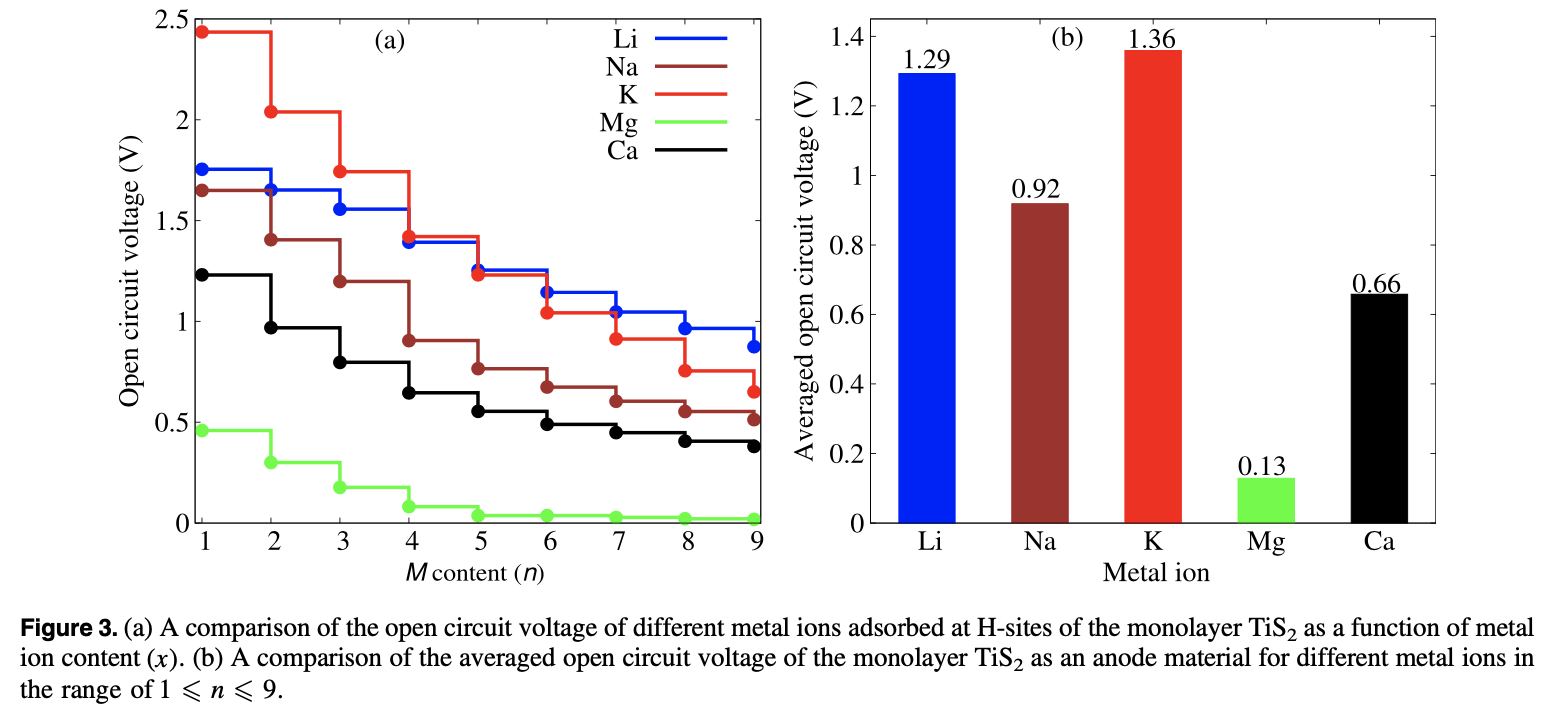
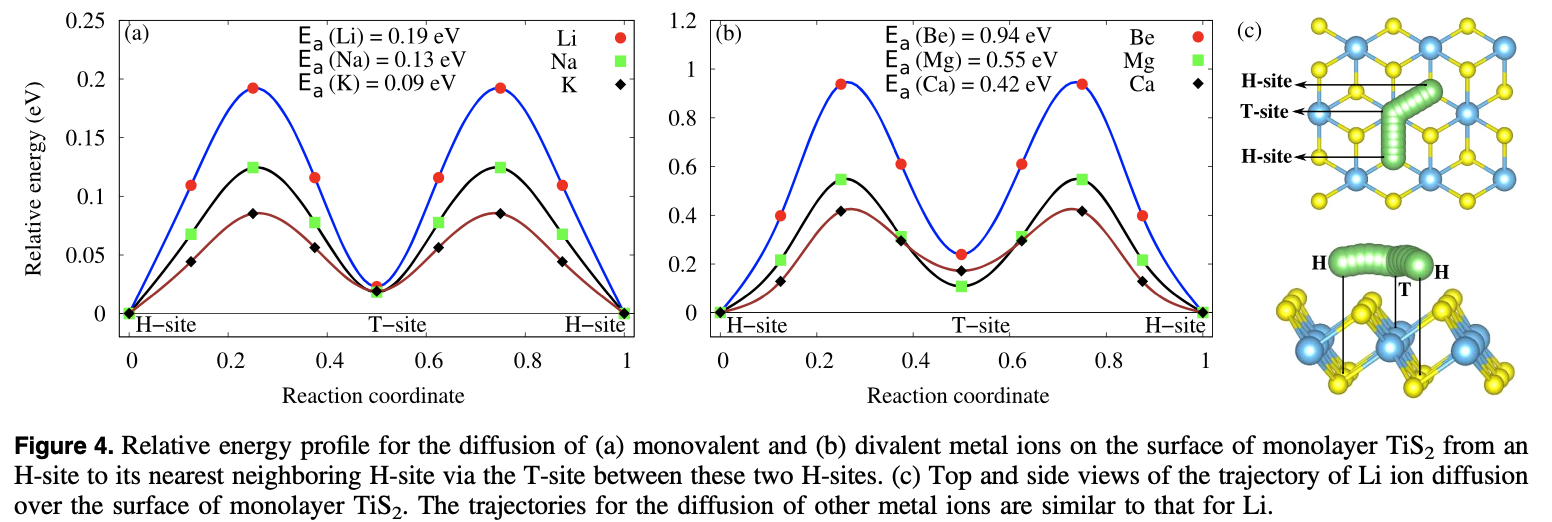
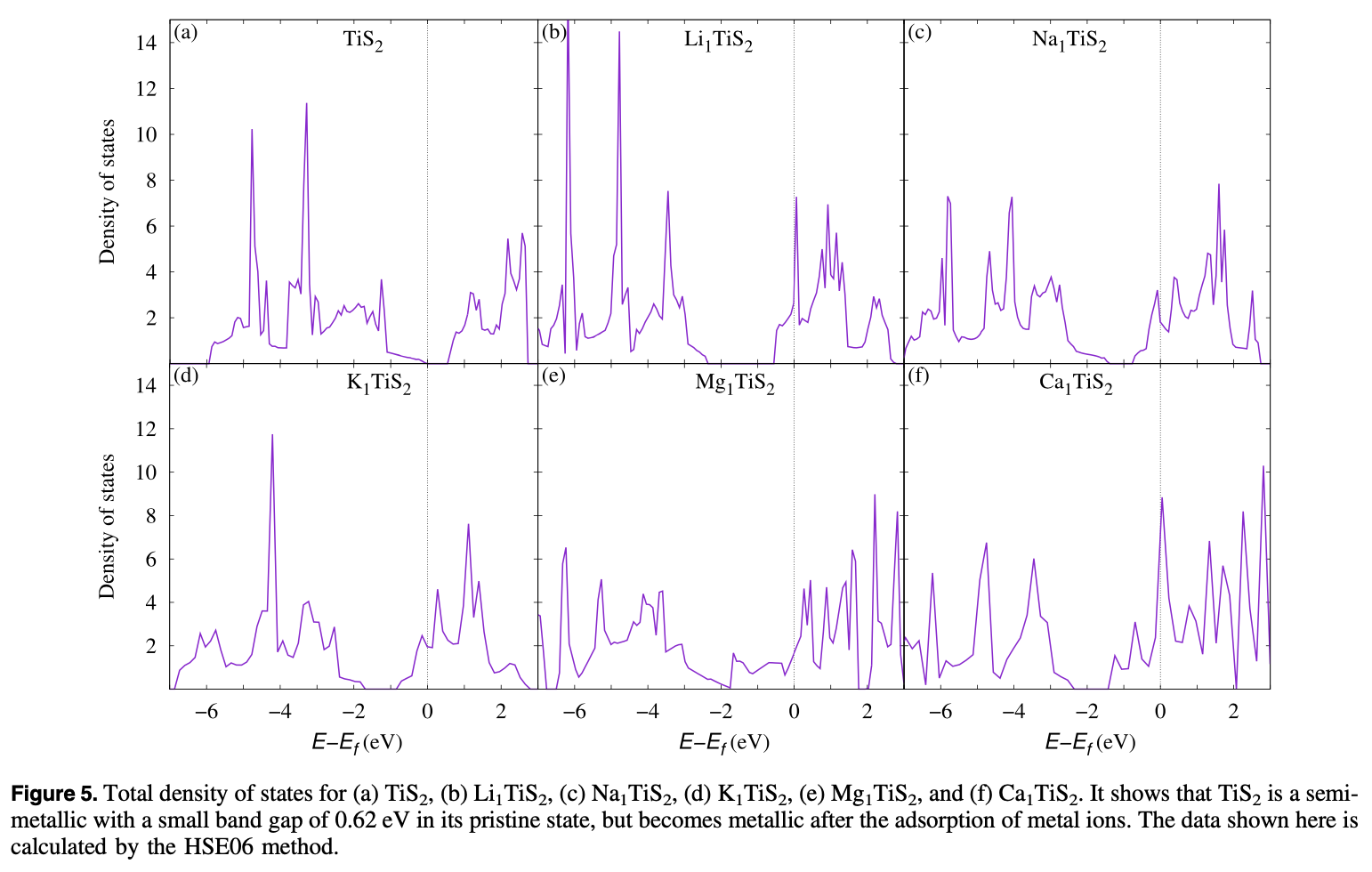
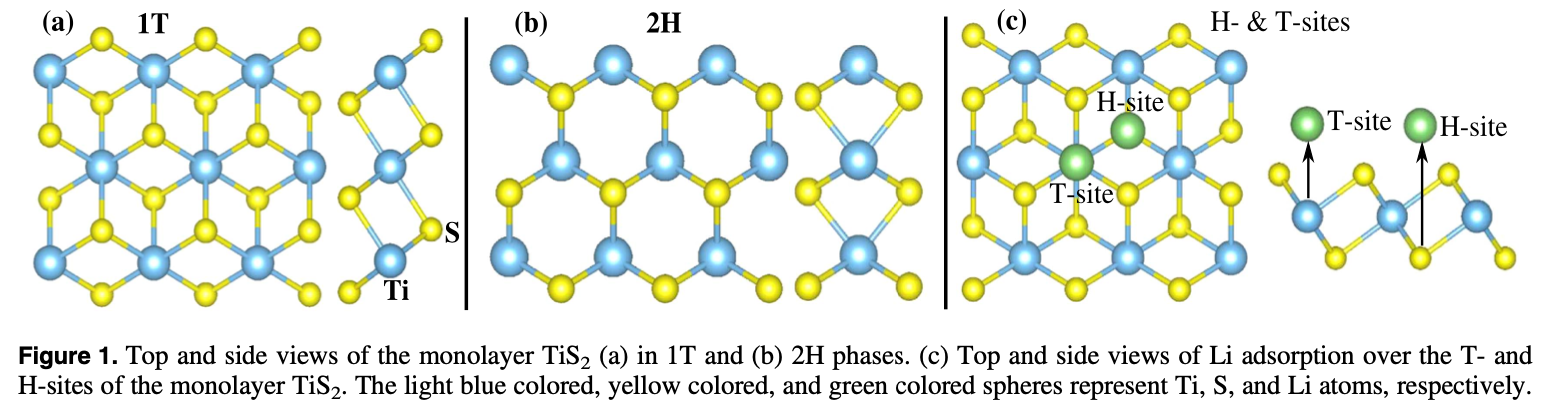
A comparative study of the monovalent (Li, Na, and K) and multivalent (Be, Mg, Ca, and Al) metal ion adsorption and diffusion on an electronically semi-metallic two-dimensional nanosheet of 1T structured TiS2 is presented here to contribute to the search for abundant, cheap, and nontoxic ingredients for efficient rechargeable metal ion batteries. The total formation energy of the metal ion adsorption and the Bader charge analysis show that the divalent Mg and Ca ions can have a charge storage density double that of the monovalent Li, Na, and K ions, while the Be and Al ions form metallic clusters even at a low adsorption density because of their high bulk energies. The adsorption of Mg ions shows the lowest averaged open circuit voltage (0.13 V). The activation energy barriers for the diffusion of metal ions on the surface of the monolayer successively decrease from Li to K and Be to Ca. Mg and Ca, being divalent, are capable of storing a higher power density than Li while K and Na have a higher rate capability than the Li ions. Therefore, rechargeable Li ion batteries can be totally or partially replaceable by Mg ion batteries, where high power density and high cell voltage are required, while the abundant, cheap, and fast Na ions can be used for green grid applications.
Authors from M3L

Aamir Shafique
aamrshafique@gmail.com
Abdus Samad
samadstar143@gmail.com