Back To Listings

- First authors: Thi Hue Pham
- Corresponding authors: Young-Han Shin
- Whole authors:
- Authors from M3L: Thi Hue Pham, Young-Han Shin
While economical and effective catalysts are
required for sustainable hydrogen production, low-dimensional
interfacial engineering techniques have been developed to improve
the catalytic activity in the hydrogen evolution reaction (HER). In
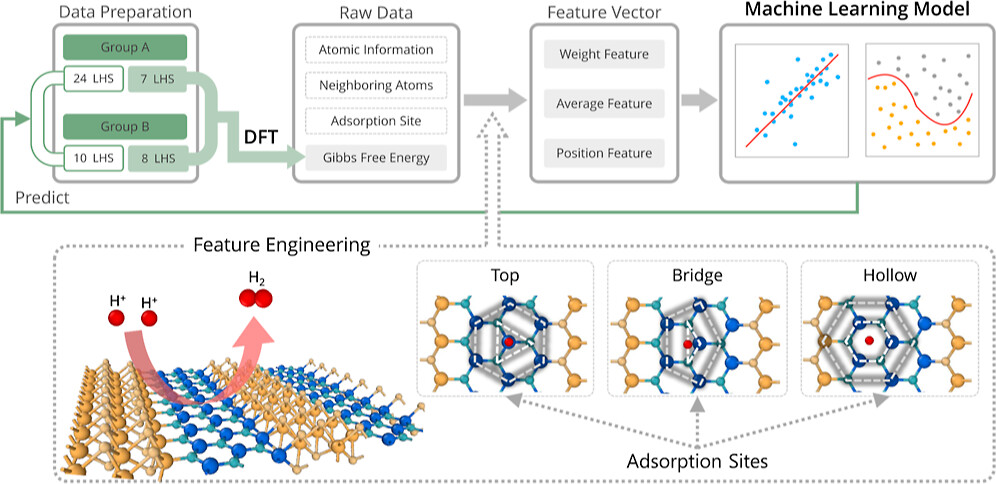
this study, we used density functional theory (DFT) calculations to
measure the Gibbs free energy change (ΔGH) in hydrogen
adsorption in two-dimensional lateral heterostructures (LHSs)
MX2/M’X’2 (MoS2/WS2, MoS2/WSe2, MoSe2/WS2, MoSe2/WSe2,
MoTe2/WSe2, MoTe2/WTe2, and WS2/WSe2) and MX2/M’X’
(NbS2/ZnO, NbSe2/ZnO, NbS2/GaN, MoS2/ZnO, MoSe2/ZnO,
MoS2/AlN, MoS2/GaN, and MoSe2/GaN) at several different
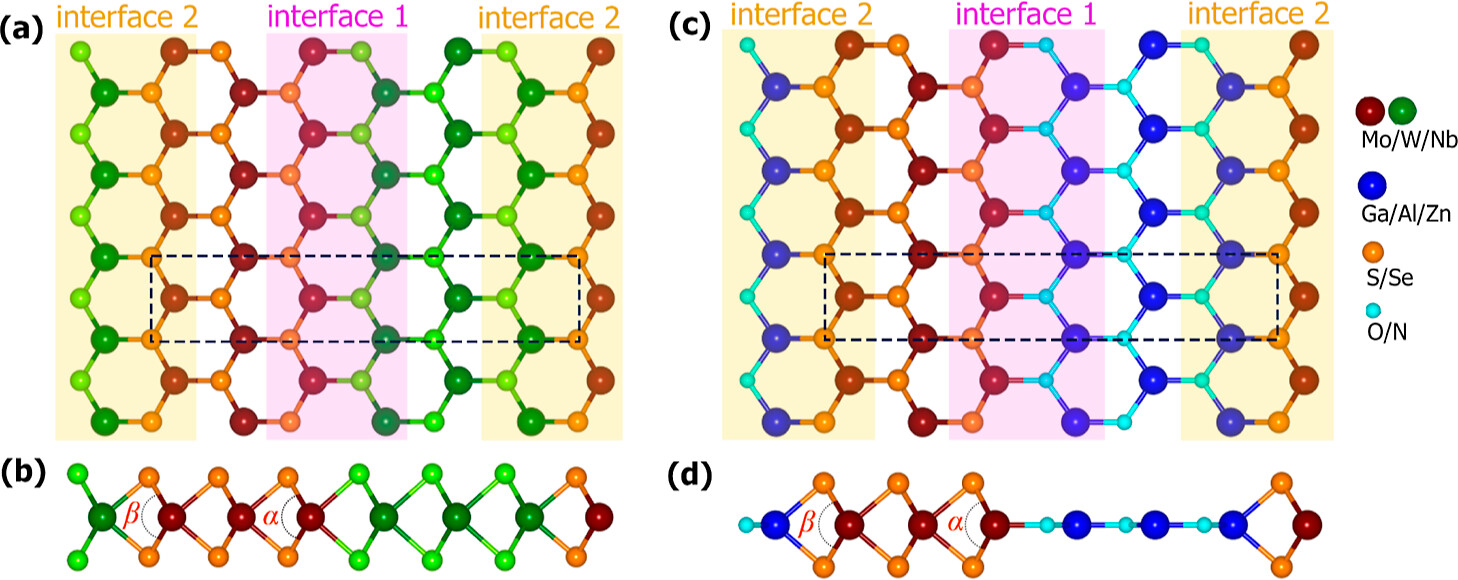
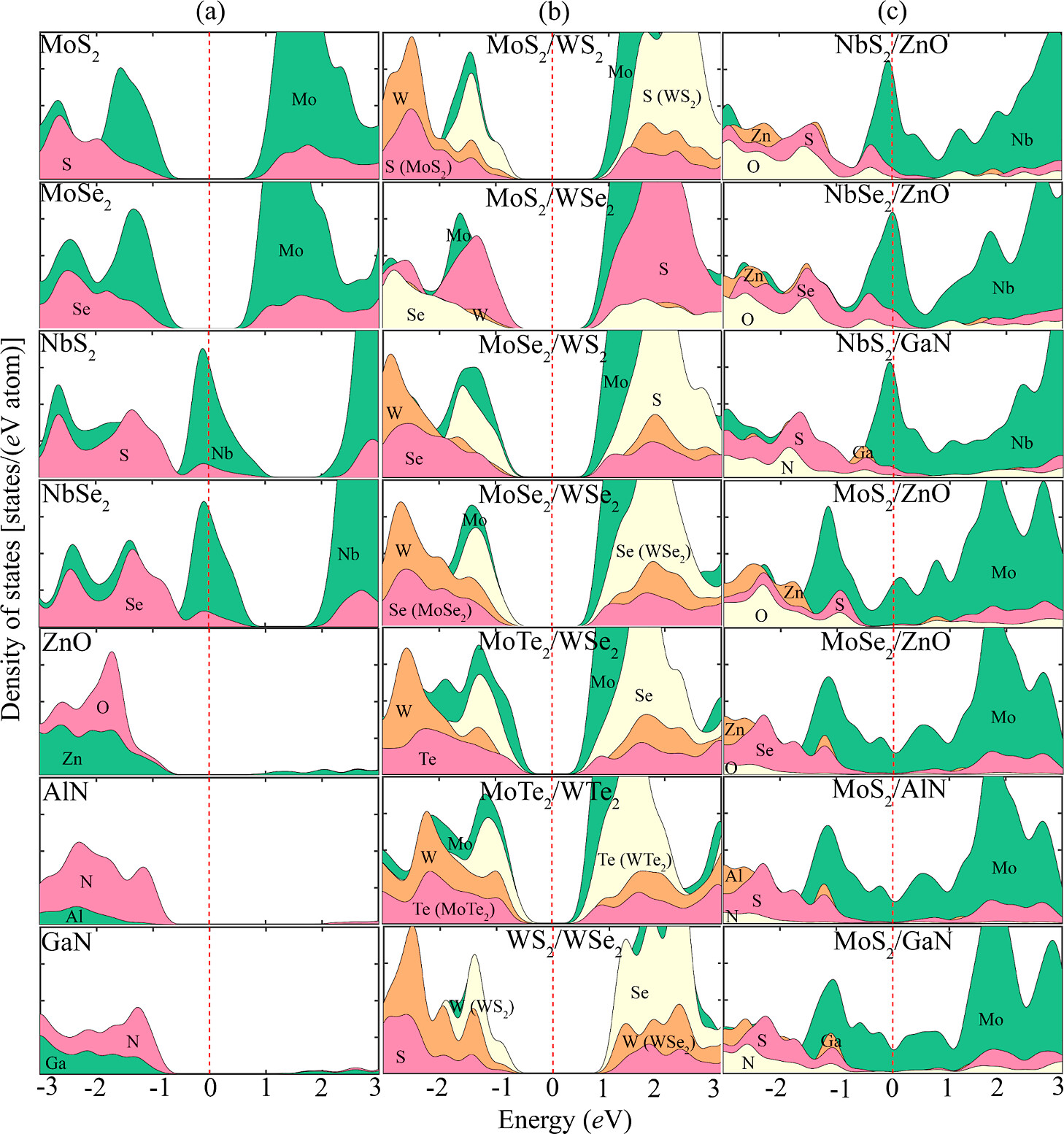
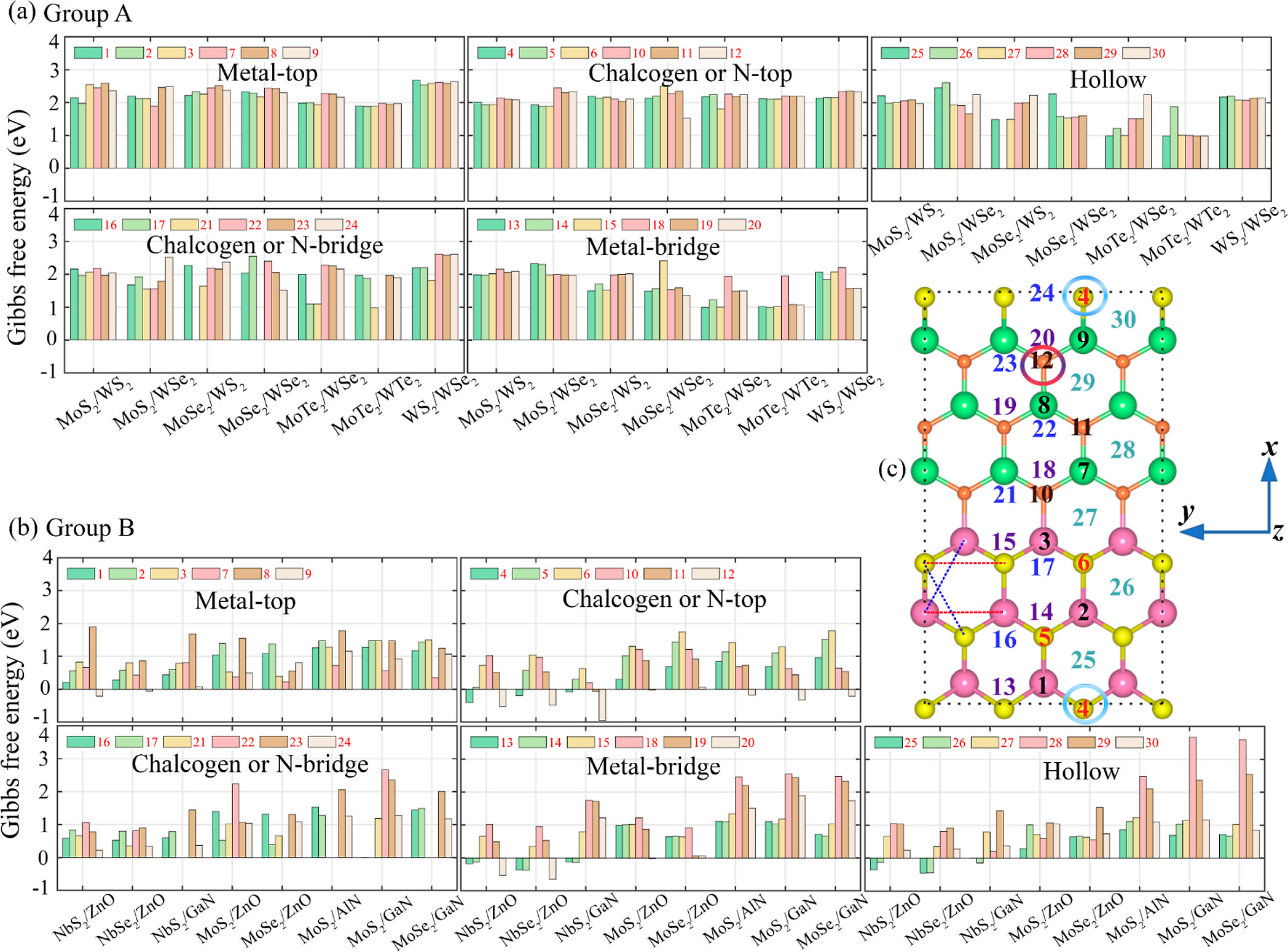
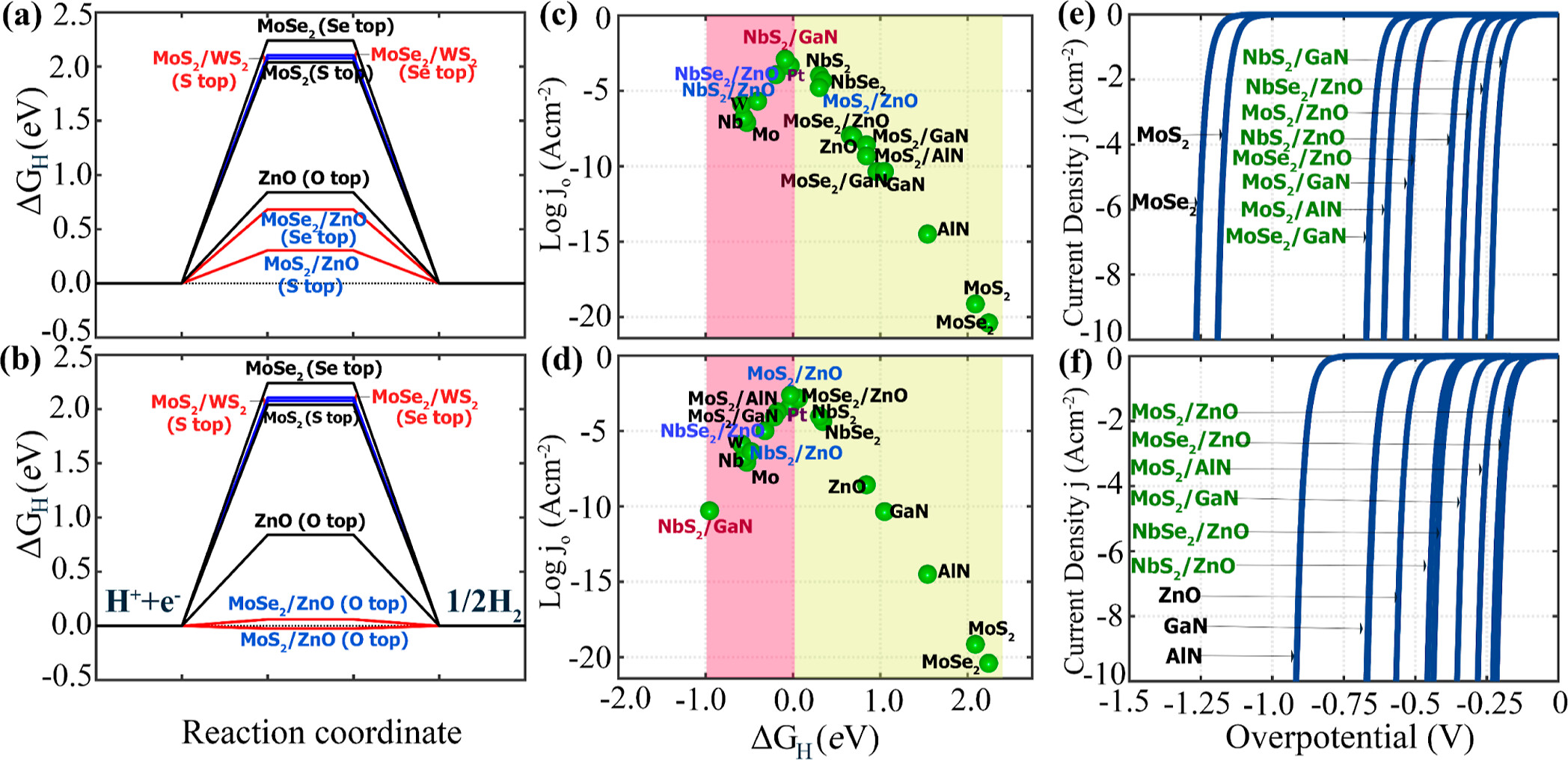
positions near the interface. Compared to the interfaces of LHS
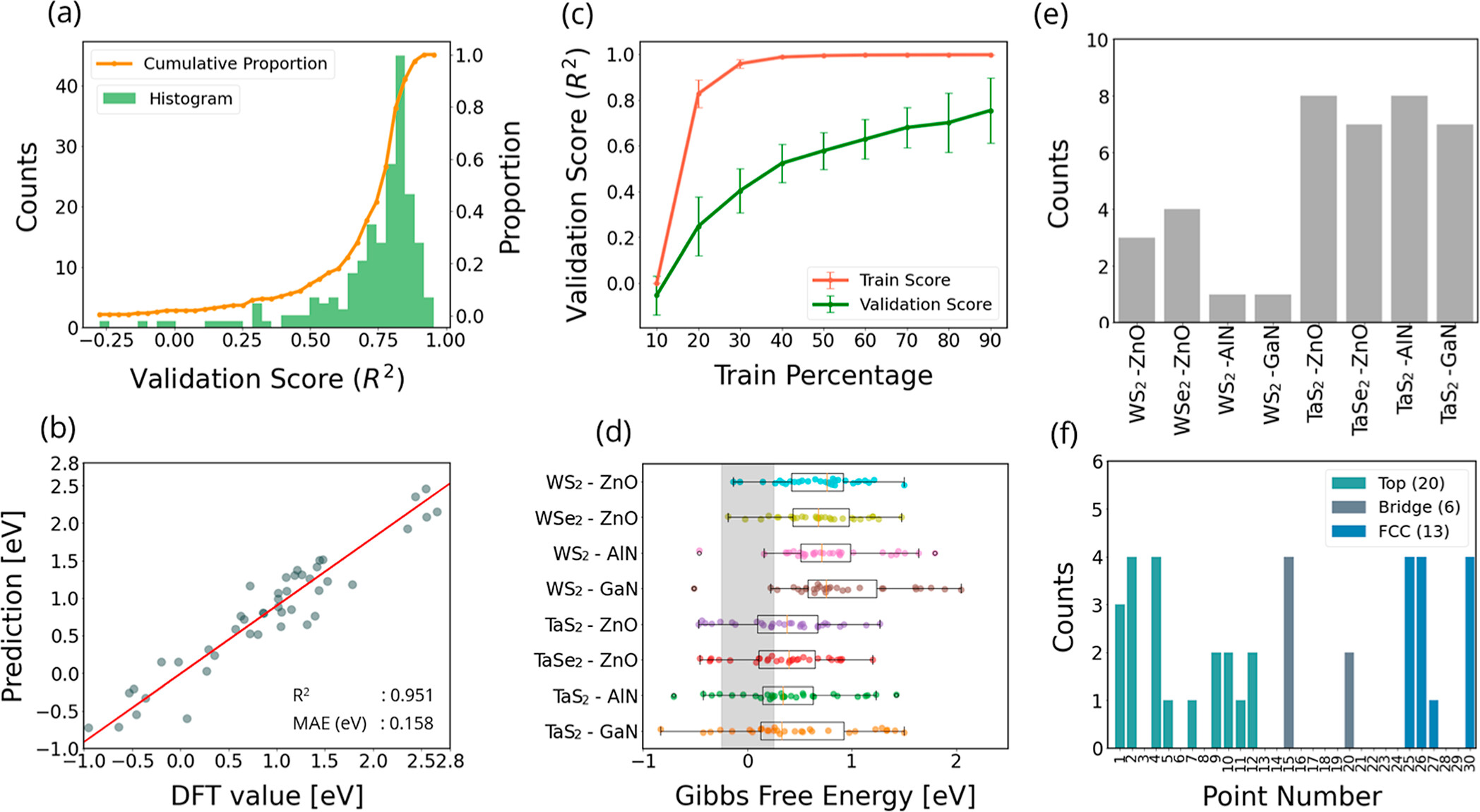
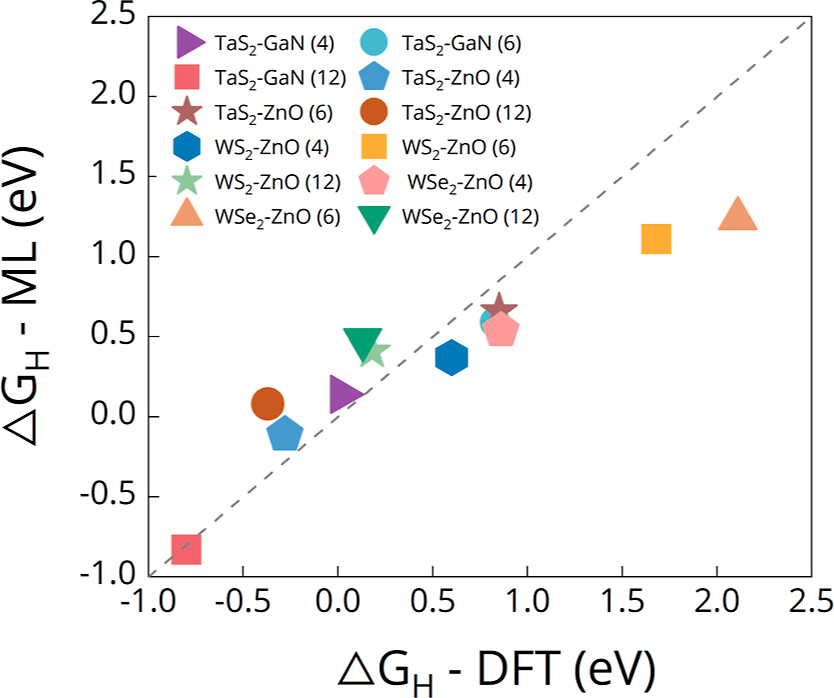
MX2/M’X’2 and the surfaces of the monolayer MX2 and MX, the interfaces of LHS MX2/M’X’ display greater hydrogen evolution reactivity due to their metallic behavior. The hydrogen absorption is stronger at the interfaces of LHS MX2/M’X’, and that facilitates proton accessibility and increases the usage of catalytically active sites. Here, we develop three types of descriptors that can be used universally in 2D materials and can explain changes in ΔGH for different adsorption sites in a single LHS using only the basic information of the LHSs (type and number of neighboring atoms to the adsorption points). Using the DFT results of the LHSs and the various experimental data of atomic information, we trained machine learning (ML) models with the chosen descriptors to predict promising combinations and adsorption sites for HER catalysts among the LHSs. Our ML model achieved an R2 score of 0.951 (regression) and an F1 score of 0.749 (classification). Furthermore, the developed surrogate model was implemented to predict the structures in the test set and was based on confirmation from the DFT calculations via ΔGH values. The LHS MoS2/ZnO is the best candidate for HER among 49 candidates considered using both DFT and ML models because it has a ΔGH of −0.02 eV on top of O at the interface position and requires only −171 mV of overpotential to obtain the standard current density (10 A/cm2).